Carbon In The Reef Tank - Part 1 (Repost)
Richard Harker wrote a two-part article that I found very helpful and easy to digest. Unfortunately as websites close down, knowledge such as his could be lost so I reached out to get his permission to store his writings here on Melev's Reef. It was written roughly 20 years ago!

Granular Activated Carbon In The Reef Tank: Fact, Folklore And Its Effectiveness In Removing Gelbstoff — Part One
One of the most frequently cited reasons given for using granular activated carbon (GAC) in a reef aquarium is to remove “gelbstoff” — the compounds that give water in a captive reef system a yellow cast. Bingman (1996) notes that “these yellow-colored compounds seem to accumulate over time” and are difficult to remove without the use of activated carbon.
As far back as 1986, Wilkens and Birkholz wrote of the use of activated carbon to “remove material that can't be biologically decomposed any further (so-called ‘yellow matter’).” Ten years later, Fosså and Nilsen (1996) echoed these feelings by writing, “the main purpose of activated carbon filters is to remove from the water any organic pigments…(that) give the water a yellow tint.”
Suppliers to the hobby have responded to the nearly universal support for the use of activated carbon by offering over two dozen GAC products. Unfortunately, the hobbyist has had very little guidance as to the functional differences between these GACs or their relative effectiveness at removing yellowing compounds.
A rare exception is an article published in Aquarium Fish Magazine by Timothy A. Hovanec (1993). Hovanec described sources of GAC and reviewed the performance of four different types of source material for carbons. Hovanec found carbon derived from bituminous coal to be the most effective in removing tannic acid from freshwater.
In a more recent article, Hovanec (1998) states flatly that “the best carbon for use in water filtration for removing dissolved organic carbons is bituminous coal-based carbon.” He also comments about the decline in use of coconut carbon, stating that “coconut carbons have the wrong pore size for filtering the target materials from water.”
Between the appearance of the first Hovanec article and the second, Stephen Spotte and Gary Adams published a study examining GAC’s ability to remove dissolved organic carbons (DOC) in a captive seawater system (1984). They reached somewhat different conclusions. They found that carbon made from hardwood was most effective in removing DOC. Carbon of anthracite coal origin was least effective.
Reconciling the conclusions of the two articles is difficult. The Hovanec study examined tannic acid removal from freshwater, whereas Spotte examined DOC removal from seawater. The two authors compared GAC from a somewhat different mix of sources (both authors examined coal based carbon, but while Spotte evaluated lignite coal based carbon, Hovanec evaluated bituminous coal based carbon). The rank order of the Hovanec study from most effective to least was coal, coconut and wood, whereas Spotte’s rank order was wood, coconut and coal — exactly the opposite. Hovanec concluded that coconut carbon was ineffective for filtering water. Spotte found coconut carbon less effective than hardwood based carbons, but still able to remove significant proportions of DOC.
Regardless of the study in which a reef keeper places more trust, Spotte identified only the manufacturer of the carbons (all were produced by one company), and Hovanec did not identify manufacturers or repackagers. (Most carbon sold to hobbyists is simply bought in bulk from a limited number of manufacturers and repackaged for the hobby. Calgon Carbon, a major manufacturer of GAC, alone produces 40 different carbon formulations.)
Potentially more useful to the hobbyist was a review of GAC written by Gregory Schiemer (1997), including several tests on 17 different brands of carbon. Unfortunately, while he examined adsorption ability, his test only subjectively measured a carbon’s ability to remove blue dye from water. Gelbstoff is composed of large complex organic compounds, so it is not clear whether a carbon’s relative performance in his tests correlates with a carbon’s effectiveness in a reef tank. While Schiemer used a five point scale to note relative adsorption, 12 of the 17 carbons rated the highest possible score. This lack of differentiation among the majority of brands makes it difficult for a hobbyist to narrow his or her choice beyond the dozen.
Test Procedure
If a carbon’s ability to remove yellowing compounds is an important reason to use carbon, it makes sense to compare the ability of various carbons to remove these compounds. Bingman (1996a) identified the source of gelbstoff as marine humic acids, and in Bingman (1996b) he noted that the presence of humic acids can be detected spectrophotometrically using the American Public Health Association (APHA) method outlined in Standard Methods for the Examination of Water and Wastewater. In this study, a Hach DR/2010 spectrophotometer was used to measure the removal of color by GAC. The one change made from the Hach procedure was to follow Bingman’s recommendation (in this case) to use a shorter wavelength of 400 nanometers (nm) rather than 455 nm as per the APHA method to increase the measurements’s sensitivity to the presence of humic acids.
The procedure consisted of circulating saltwater containing gelbstoff through a quantity of carbon for 12 hours and determining the reduction in color over the time period. My 300 gallon reef tank water, even after several months without GAC, measures only 9 APHA color units, so a method outlined in Sieburth and Jensen 1969) was utilized to increase the color of the water. A combination of Calerpa and Sargassum algae was allowed to “stew” for several hours in warmed water removed from my tank until sufficient materials had been exuded from the algae. The exudations of algae are a combination of phenols and carbohydrates that bind with proteinaceous matter in saltwater to produce the yellowing compounds. This approach raised the apparent color from 9 to 89 units in a day, enough for the color to be visually detected against a white background.
Twenty grams of GAC were sandwiched between two layers of plastic filter material supported by 38 grams of polyester filter floss in an 8 by 30 centimeter vertically oriented acrylic tube. This arrangement forced water to flow evenly through the carbon. The quantity of carbon was chosen to reflect the recommendations of hobby authors regarding the use of carbon.
The carbon chamber was gravity fed with a powerhead moving approximately 1.5 liters per minute, pumping water to the top of the chamber. The chamber was placed in a tank filled with 4 liters of test water. While hobby authors generally give recommendations on the quantity of carbon to use, none of the sources I reviewed offered opinions on the rate at which water should flow past carbon. Hovanec offered no details on the flow rate of his experiment. Spotte and Adams conducted their flow-through experiment at 24 milliliters/minute (ml/min), considerably slower than my rate. Other work suggests that the effectiveness of GAC begins to decline with flow rates higher than 65 ml/min (Kerr and Quinn).
The traditional method of GAC use in a reef tank is to repeatedly circulate water through a canister filter or PVC tube fed by a powerhead (Moe), and both methods involve much higher flow rates. For this experiment, it was decided that rates closer to hobbyist rates would be utilized.
Color was measured at the beginning of the 12 hour test period, at least once during the test, and then once again at the end of the period. For each measurement, three replicates of approximately 30 ml each were removed from the test tank and then successively poured into a 1-inch cell. The color of each replicate was then measured with the spectrophotometer. The three measurements were averaged for the final result listed in the chart below. The blank for each test consisted of distilled water.
Results
All GACs were able to remove some color, but effectiveness varied considerably between brands. The highest reduction was 88 color units, virtually eliminating any color, while the lowest was 21 units, only a slight reduction over the initial color of the water. A control test using only the polyester filter floss removed 7 color units. Three of the top five performing carbons were lignite based. The top five carbons adsorbed considerably more color than the second tier of bituminous based carbons. The lone coconut based carbon ranked eighth, outperforming several bituminous coal-based carbons.
As a rule, the molded “pellet” type GACs were less effective in removing color than the other brands. Three of the four lowest adsorbing carbons were pellet shaped. The pellets had smooth, hard surfaces. The most effective carbons tended to be irregular in shape and offer a large surface area to volume. The surfaces of the more effective carbons were rough rather than smooth like the pellet carbons.
While there are many brands of carbons sold to hobbyists, there are a limited number of types of carbon sold. Bituminous coal based carbons make up the majority of brands available. Lignite based carbons make up the rest with the exception of a few coconut based brands. This may have affected the outcome of the tests. For example, only one coconut carbon was tested, and it was a mid-pack performer. It did, however, outperform the same company’s “scientific grade” bituminous based carbon. This suggests that had I been able to acquire more formulations of coconut, coconut based carbons may have performed better.
Hovanec (1998) writes that “(coconut carbon) was the subject of much marketing hype…but the drawbacks of using coconut carbon in the aquarium finally merged with the market forces and sales of this type of carbon dropped.” One wonders what marketing forces would have prevailed if Spotte’s findings had been as widely read as Hovanec’s. Perhaps the hobbyist would have a greater range of choices available to him. None of the large repackagers sell hardwood based carbon to the hobby, so it was impossible to confirm Spotte’s findings regarding the effectiveness of hardwood GAC.
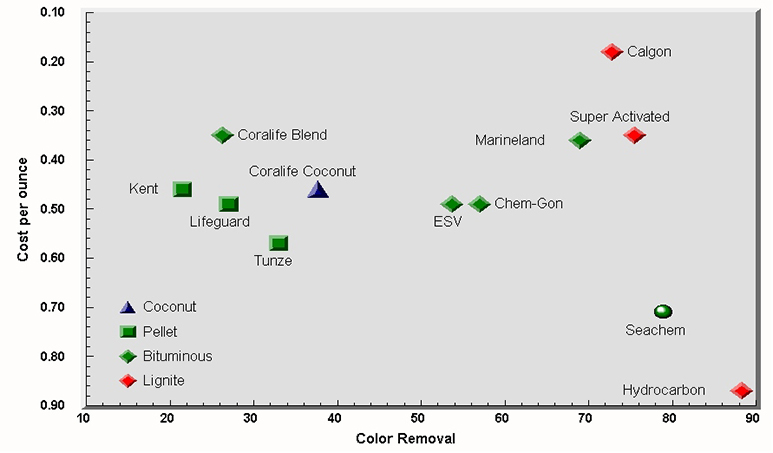
GAC available to the hobby varies considerably in price, but price is a poor predictor of effectiveness. Some of the least expensive GACs outperformed GACs costing nearly three times as much. Figure 1 shows the relationship between effectiveness in removing color and efficiency expressed in cost per ounce. GACs shown in the upper right quadrant effectively remove color, and do so at a low cost per ounce. GACs in the lower right quadrant also effectively remove color, but do so at a higher cost. The GAC's found in the left quadrants are less effective on a unit cost basis than those shown to the right.
In part two of this examination of GAC we’ll explore the use of carbon in captive reef systems. We’ll examine the recomendations offered to hobbyists on how to use GAC and look at the growing trend of passive use of carbon.
Acknowledgments. I wish to thank Jeff Voet of Tropical Fish World of Raleigh, North Carolina for his assistance in selecting and procuring many of the GACs used in this review. References will be included next month in part two.












